Lignin and Its Derivatives
Properties
Lignin is the second most abundant renewable biopolymer found in nature, with celluose being the most abundant. It is a relative complex compound having a cross-linked phenolic-type structure which does not easily breakdown. Due to its aromatic structure, it is more chemically stable and heat resistant than cellulose.
Lignin is of vital importance as a structural material in wood and can account for up to 40 % of the dry biomass.1 It is basically the aromatic part of the wood "composite" that acts like an adhesive in plant cell walls and provides rigidity to the wood. It also prevents rapid break down of the cellulosic material since it is mostly found between cells, but also within the cells itself.
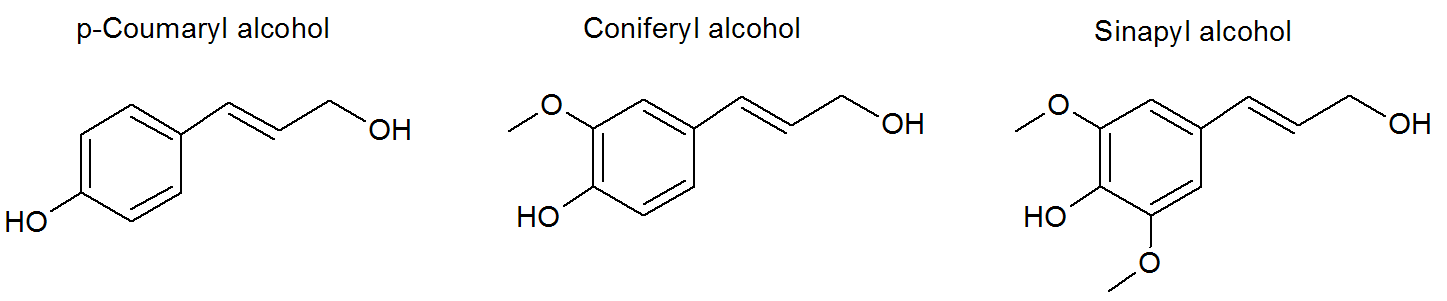
Lignin is a cross-linked biopolymers with a moderately high molecular weights of more than 5,000 g/mol (weight average2). The true degree of polymerization is difficult to measure since the polymer tends to fragment during extraction. The extract consists of various types of lignins depending on the pH and extraction process.2 Unlike cellulose and hemicellulose, the chemical structure of protolignin or native lignin cannot accurately defined. However, the major building blocks (the so called monolignols are well known. The three most important monolignols found in lignin are p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol which are incorporated into the lignin macromolecule as p-hydroxyphenyl (H), syringyl (S) and guacyl (G).3
Lignin is one of the most stable biopolymers. New developments focus on the increase of lignin's biodegradation rate through incorporation of other molecules into the lignin structure.
Lignin and its derivatives are effective antioxidants and can be used in plastics such as polyolefins and cellulose derivatives as well as in many formulated products like food, pharmaceuticals and cosmetics (food-grades). Lignins radical scavenging ability is not the same for all fractions but depends on the hydroxyl content, molecular weight and solubility among various other factors. A very effective antioxidant is calcium lignosulfonate.4
Synthesis
Lignin is a byproduct of the bleaching process of wood pulp. The goal of this process is to remove all or most of the lignin in the wood pulp because residual lignin in cellulosic materials has a negative impact on the cellulose properties. Thus it has to be modified, degraded or completely removed from the cellulose
to produce high quality paper products. The pulping process usually
includes following steps: 1) debarking and chipping; 2) mechanical
and/or chemical separation of the wood fibers by grinding,
refining or digestion (cooking) to dissolve the lignin; 3) removal
of discoloration (primarily residual lignin)
by bleaching; and 4)
cellulose processing and paper manufacture.
To remove lignin and hemicellulose and to produce high quality cellulose, the wood pulp is treated with a alkali sulfite or bisulfite at temperature above 130°C in a large pressure cooker, the so called digester, which hydrolysis the lignin and makes it water soluble (sulfite process). A competing chemical pulping process is the so called sulfate or Kraft process which is the dominant chemical pulping process today. In the Kraft pulping process, the wood chips are soaked in white liquor which is a hot mixture of water, sodium hydroxide, and sodium sulfide. The chips are then cooked (digested) at around 170°C to hydolyse and dissolve the lignin. After cooking, the raw pulp is screened, washed, and most of the process water is removed. The raw celluose (brown stock) still contains noticable amounts of lignins which cause discoloration. To produce light colored or white paper, the wood pulp is bleached which causes breakdown and modification of the remaining lignin. A number of bleaching agents can be used and are sometimes applied in a stepwise manner within a bleaching sequence. Common bleaching agents include hydrogen peroxide, ozone, potassium peroxymonosulfate, sodium hydrosulfite, sodium peroxide, chlorinedioxde (NaClO3 + H2SO4 + SO2 → 2ClO2 + 2 NaHSO4), chlorine hypochlorite (Cl2 +H2O ↔ H+ + Cl- + HClO) and peroxyacetic acid. A modern bleaching process starts with treating the pulp with oxygen and ozone. The bleached pulp is then washed with sodium hydroxide and treated in sequence with alkaline peroxide and sodium dithioate (sodium hydrosulfite). The reaction of lignin with these compounds leads to fragmentation and chemical modifications of the lignin. One of the major byproducts of the sulfite process are lignosulfonates or sufonated lignins. These compounds are rather inexpensive and are used for a variety applications.
The composition of technical lignin varies greatly with the type of raw material, delignification process and pulping conditions. Only mechanical pulping leaves the lignin and the cellulose intact whereas sulfite/sulfate treatment and bleaching leads to breakdown and chemically modification of the lignin.
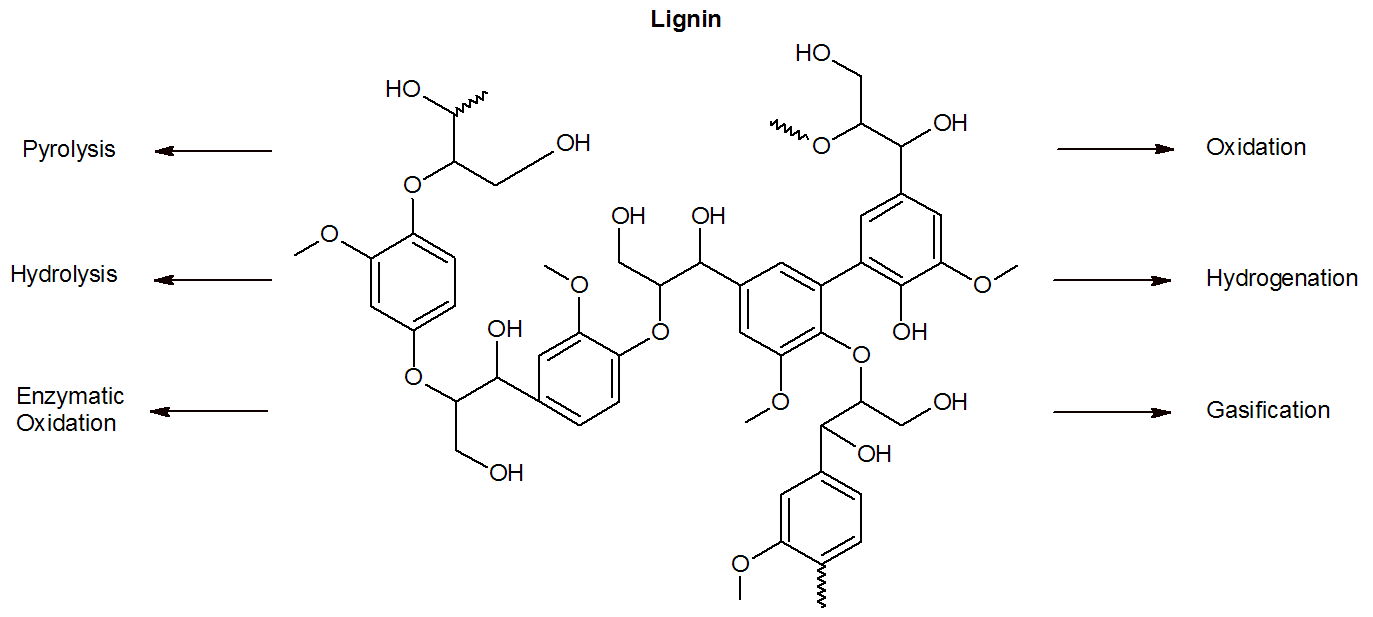
Due to the large number of reactive groups in the lignin, a large number of different products can be made. In a first step the crosslinked polymer has to be broken down. Since the carbon- carbon bonds are very resistant to chemical attack, the fragmentation is mostly limited to the ether linkages. Important processes that produce (higher value) lignin derivatives include oxidation (Vanillin, DMF, DMSO), (enzymatic) hydrolysis (biofuel), and pyrolysis (methoxy-substituted phenols and cresols).
One of the most important (high value) products dervided from sufonated Lignin is artificial vanillin. However, most of todays synthetic vanillin is synthesized from petrochemicals.
COMMERCIAL Lignin Products
Today, high quality lignin is commercially available in large quantities which is converted to numerous other raw materials which have been fractionated, purified, chemically modified (functionallized). The most important types of lignin include soda lignin, Kraft lignin, hydrolyzed lignin, organosolv lignin and lignosulfonates. Major manufacturers of these products are Alberta Pacific, Borregaard LignoTech, CIMV, Domtar, Domsjö, Tembec, UPM, and Weyerhaeuser among several other companies.
APPLICATIONS
Lignin based materials are used on a much smaller scale than cellulosic materials. However, sales of lignin and its derivatives have steadily grown.
Lignosulfonates are the most important pulping byproducts of the sulfite process. They are used as additives in many products such as oil well drilling fluids (rheology modifier), concretes (strength aid, superplasticizer, retarder), drywalls (water reducing agent), agricultural chemicals, and animal feed (binder). Lignosulfonates are also used as dispersant agents (dyes), complexing agents and emulsifying agents. One of the most important applications of Lignin derivatives (ligno-sulfonates) is the production of oriented strand, fiber, and particle boards. Lignin derivatives are added to many wood glues such as phenol-formaldehyde, urea-formaldehyde, melamine-formaldehyde, resorcinol-formaldehyde and tanin-formaldehyde resins. They are mainly added to reduce raw material cost.
Kraft lignin is the main byproduct of the Kraft pulping process. This form of lignin has very different chemical properties than lignosulfonates; since no sulfonate groups are present, Kraft lignins are only soluble in alkaline solution above pH 10. Due to the low reactivity, Kraft lignins find only niche applications and the bulk of it is burned providing energy to run the paper mill.
There are numerous other (potential) applications for chemically modified and functionalized lignin. For example, Lignin derivatives could be a cheaper alternative to existing petroleum based raw materials in a variety of products such as coatings, paints, plastics, resins (plasticizer) and liquid fuels. Since lignin and its deriviatives are natural antioxidants they are also useful to protect various products.4
1M.P Pandey, C.S Kim, Chem. Eng. Technol., 34, 1, 29-41 (2011)
2P. Ma, Y. Gao, and H. Zhai, Bio Resources, 8(4), 5581-5595 (2013)
3R. Vanholme, B. Demedts, K. Morreel, J. Ralph, and W. Boerjan, Plant Physiology, Vol. 153, pp. 895–905 (2010)
4G.L. Catignani, M.E. Carter, J. of Food Sci., Vol 47, 1745 (1982)